How to consider sex and gender in trials
Beyond sex-specific organs, there are numerous disease and treatment-related differences between sexes. Unfortunately, historically, research has heavily relied on male participants, leading to the common practice of extrapolating results and assuming applicability to female patients. This approach can be misleading and potentially harmful with female patients experiencing higher rates of adverse drug reactions and having limited options when pregnant. We have written an editorial on the importance of considering the potential differences in treatment effect by sex – Another Brick in the Wall … no More! Breaking the Sex Bias.
It is important to draw a distinction between sex and gender. The two terms should not be used as synonyms, especially when designing clinical trials.
- Sex refers to the biological characteristics observed in humans and animals, encompassing various factors such as chromosomes, gene expression, hormone function, and reproductive/sexual anatomy. These attributes typically lead to the classification of individuals as female or male, although there can be variations in the specific biological traits that constitute sex and how these traits are expressed in different individuals.
- Gender refers to the socially constructed characteristics of women, men, and gender-diverse people such as norms, roles, behaviours, and identities irrespective of their genetic make-up.
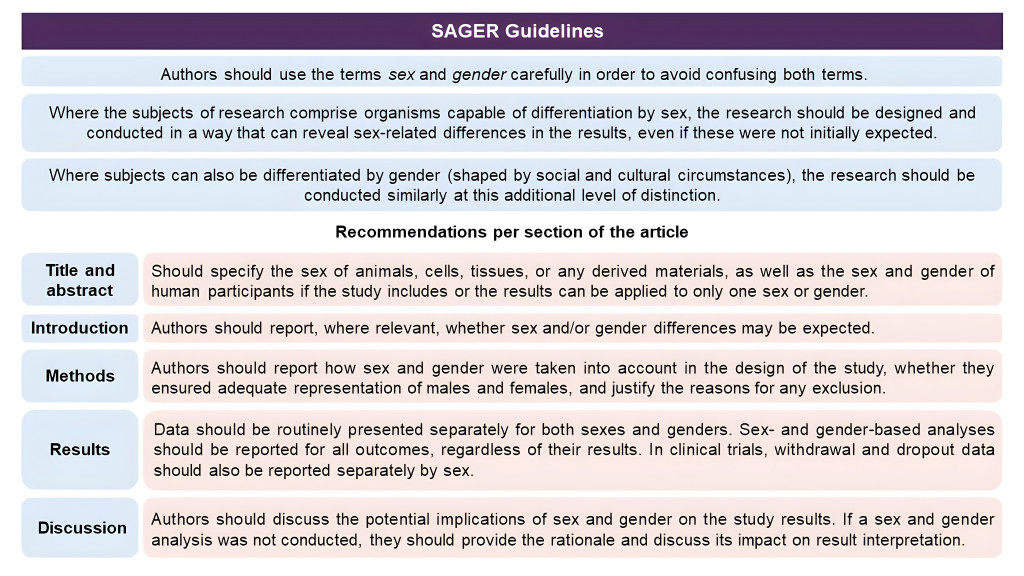
To facilitate sex and gender equity in research The European Association of Science Editors has developed The Sex and Gender Equity in Research (SAGER) guidelines. A panel of 13 experts representing nine countries worked on the guidelines by surveying journal editors, scientists, and other members of the international publishing community and conducting a literature search. The result is a comprehensive procedure for reporting sex and gender information in study design, dana analyses, results, and interpretation of findings.
A short summary of the guidelines can be seen in the image below. More information, including a SAGER guidelines checklist can be accessed here.
The UK Medical Research Council (MRC) also provides diversity guidance for researchers covering aspects like sex in experimental design, available here. This is part of MRC’s policy that requires all applicants for MRC funding to consider diversity and inclusion, including many of the points outlined in the SAGER guidelines.
If you are looking for guidance on how to ask questions on sex and gender, Equality, Diversity and Inclusion in Science and Health have developed question guidance available here.