How to collect ethnicity data
The INCLUDED (INCLUsivity through improving the practice anD utility of Ethnicity Data collection in trials) project aimed to find out how data on ethnicity are being collected and handled in clinical trials, what those from diverse ethnic groups think about this, whether this has any bearing on their participation in research, and how the data collection and handling could be improved.
WHAT DID WE KNOW BEFORE WE BEGAN THE STUDY?
Ethnicity data are essential for appropriate treatment and for equity. People from diverse ethnic communities are under-represented in clinical trials.
What did we do?
We asked Clinical Trials Units in the UK how they collected ethnicity data in their trials and also consulted several trials-related forums. Twenty-four Clinical Trials Units responded, providing information about 41 recent trials. We also conducted nine focus groups or interviews with those from diverse ethnic communities to find out how people feel about the collection of their ethnicity data, how this might affect whether they take part in trials and what improvements they could suggest. We presented our findings and draft recommendations to people with some interest in the results, including trialists not involved in the research, researchers working on inclusivity, funders, and members of diverse ethnic communities. Following their comments, we produced 13 recommendations.
What did we find out?
Diverse ethnic communities felt that for the trial to be both robust and inclusive, it was important to be meaningfully involved in developing plans for ethnicity data collection at an early stage, but researchers from Clinical Trials Units told us it was rare that they did this.
Researchers in Clinical Trials Units and participants from diverse ethnic communities were not always certain why ethnicity data had been collected in trials they knew about. Researchers also expressed uncertainty about how to connect with those in diverse ethnic communities.
Currently, clinical trialists almost always use a pre-specified list of ethnicities to collect data about ethnicity. However, there is a lack of consistency between trials in the lists being used. This is despite the common belief that using such a list improves consistency. People from diverse ethnic communities feel that their ethnicity is often not accurately represented in these lists. This can feel discriminatory and exclusionary.
Alongside the problems related to pre-specified lists and the use of ethnicity data not being properly explained, those from diverse ethnic communities explained that historic abusive research and current discriminatory attitudes they experienced in health care influenced how they felt about participating in research.
We also discovered that ethnicity is not easy to define but we liked this explanation from a paper in 2004 by Raj Bhopal, as it reflected our thinking about the fluid and multi-faceted nature of ethnicity:
“Ethnicity is a multi-faceted quality that refers to the group to which people belong, and/or are perceived to belong, as a result of certain shared characteristics, including geographical and ancestral origins, but particularly cultural traditions and languages. The characteristics that define ethnicity are not fixed or easily measured, so ethnicity is imprecise and fluid. Ethnicity differs from race, nationality, religion, and migrant status, sometimes in subtle ways, but may include facets of these other concepts.”
Raj Bhopal, 2004
What do we recommend?
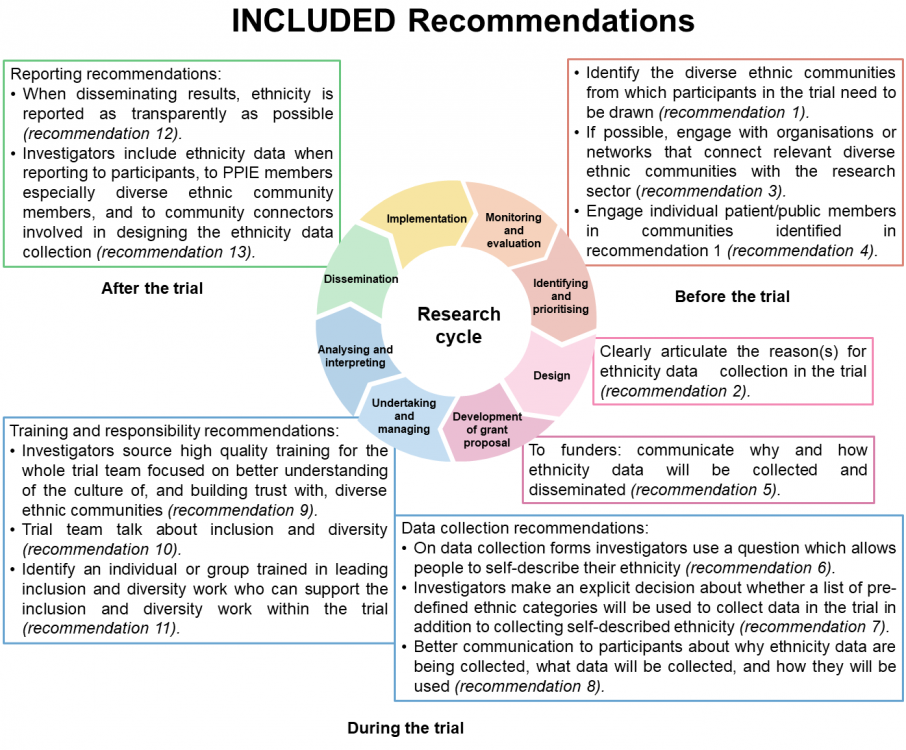
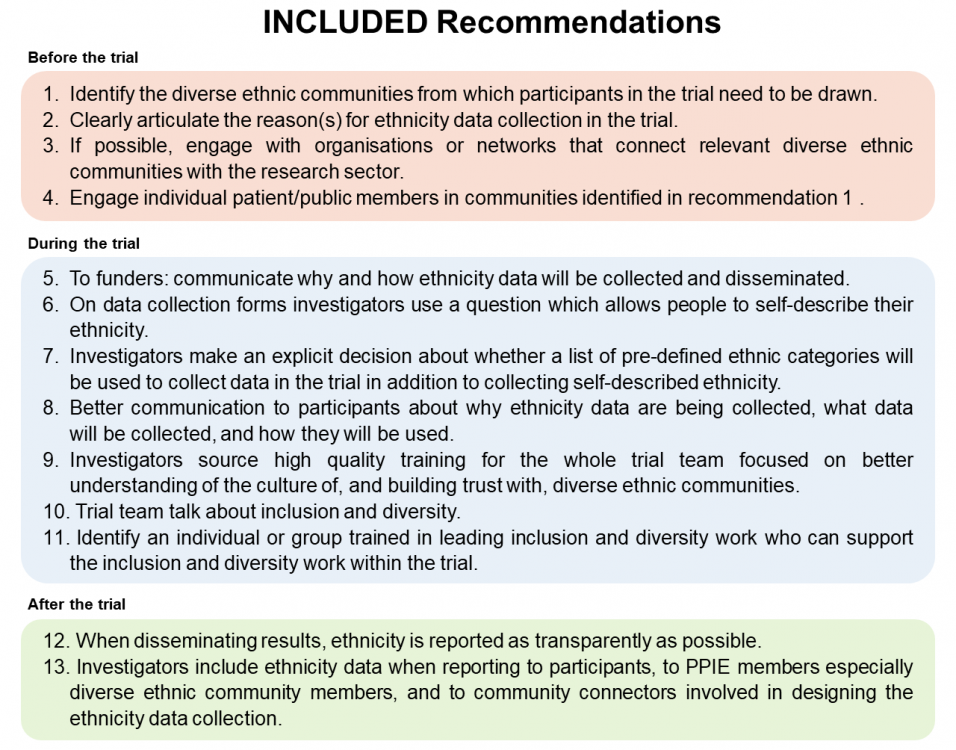
The diagram below gives an overview of the 13 recommendations (or download as a Powerpoint). We’ve given a summary of the recommendations as a table too.
You can also download a short summary document containing a little more detail on each recommendation.
These are v1.0 of the recommendations and we’re releasing them because a) we think they might be useful but also b) because we are keen to get comments and feedback on them sooner rather than later. If you’d like to get in touch, please contact the lead researcher Professor Sandra Eldridge s.eldridge@qmul.ac.uk.
Who was involved in this research?
Researchers came from Queen Mary University of London, and the universities of Keele, Aberdeen, Oxford, and Edinburgh. Community partners in the research were Egality Health, Social Action for Health, South Asian Health Action, Caribbean and African Health Network, Race Equality First, and a Latin American group. A Public Advisory Group connected with Edinburgh University advised throughout.
Where can I find out more?
For more information please contact the lead researcher, Professor Sandra Eldridge s.eldridge@qmul.ac.uk.
FUNDING
This work been funded by the National Institute for Health and Care Research.