Instead of giving people the choice to opt-in a study, they are given the choice to opt-out.
Opt‐out consent may improve recruitment.
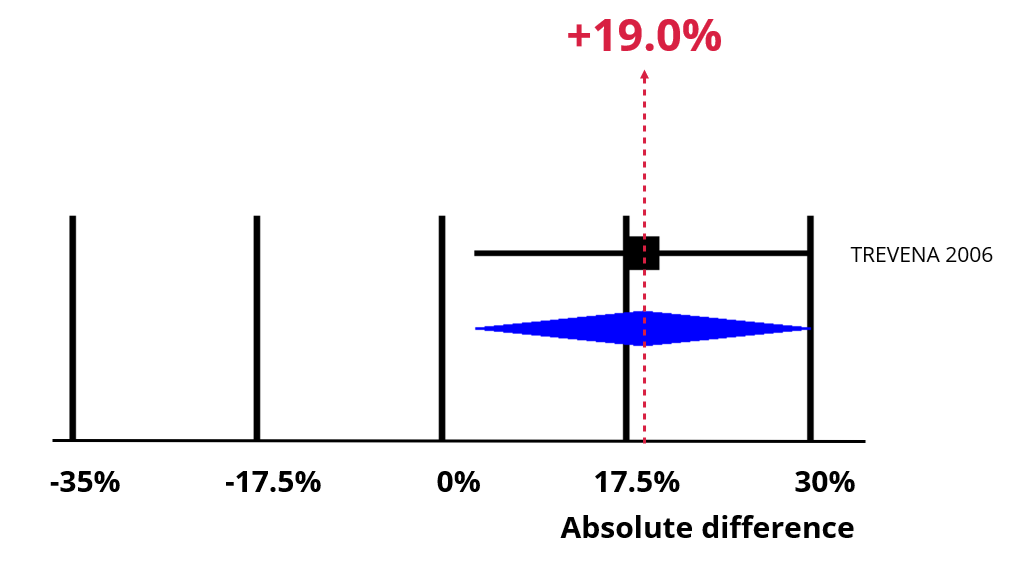
An increase of 19% (95% confidence interval = 3% to 35%).
GRADE Low certainty.
We recommend that trialists consider using opt-out rather than opt-in consent.
See Resource bundle below for details on how to use opt-out consent.
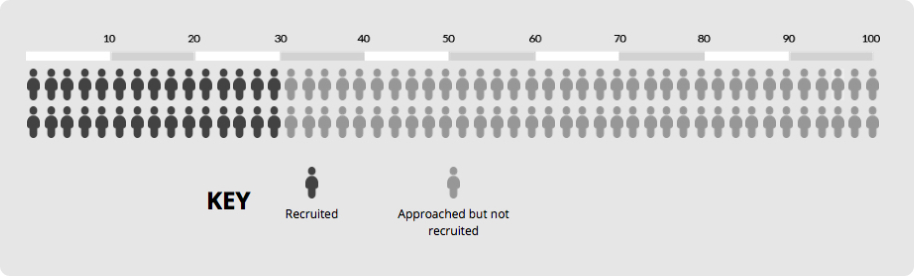
Imagine a trial that needs to recruit 30 participants and initial recruitment is 30% of those approached. This means you’d need to approach 100 people to recruit 30 of them (see chart).
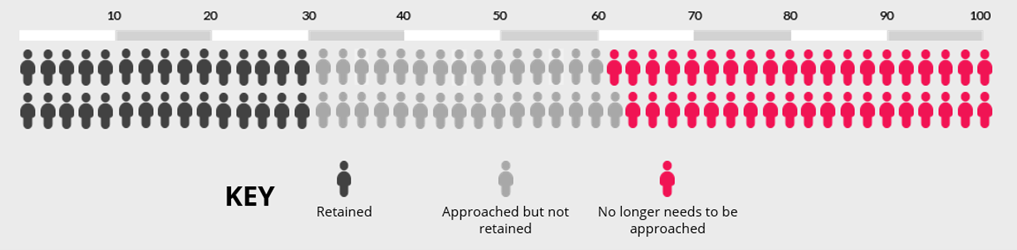
Now imagine using opt-out rather than opt-in consent. The chart below shows the impact of an absolute increase of 19% (95% CI = 3% to 35%). Recruitment is now 49%, which means our best estimate is that 61 people would now need to be approached to recruit 30 of them.
Trial Forge will make trials more efficient by looking for marginal gains across all trial processes, from research question to implementation into routine care. It will encourage everyone connected with trials to be more sceptical of what we do by asking for the evidence behind all of our trial decisions.