A more patient friendly patient information leaflet is developed using user feedback (rewritten for a lay audience, reorganised into subsections, had graphic design and undergone user feedback).
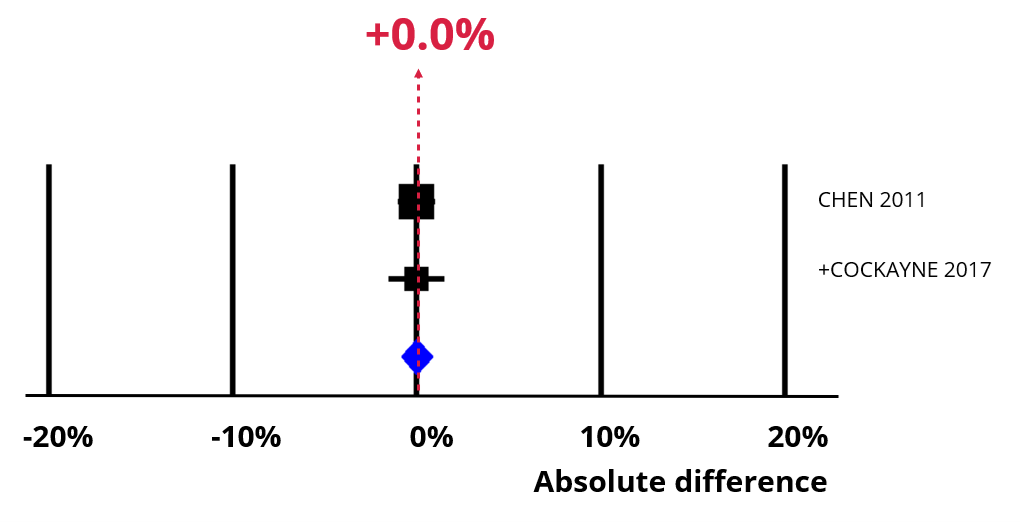
Optimising the PIL through using user feedback probably makes little or no difference in recruitment.
An increase of 0% (95% confidence interval = 0% to 1%).
GRADE Moderate certainty.
We recommend that trialists develop patient information leaflets with user feedback in the context of an intervention evaluation
See Resource bundle below for details on how to develop patient information leaflets with user feedback.
Imagine a trial that needs to recruit 30 participants and initial recruitment is 30% of those approached. This means you’d need to approach 100 people to recruit 30 of them (see chart).
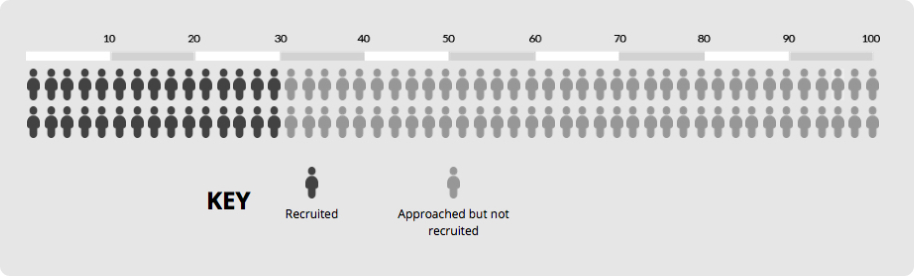
Now imagine using a participant information leaflet developed using feedback. The chart below shows the impact of an absolute increase of 0% (95% CI = -2% to 2%). Recruitment is still 30%, which means our best estimate is that 100 people would still need to be approached to recruit 30 of them.