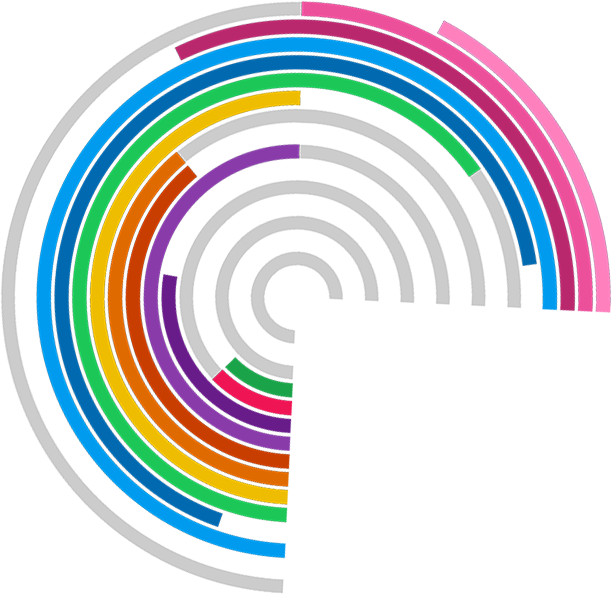-
 A systematic approach tomaking trials more efficientThe evidence base for how to make the trials process
A systematic approach tomaking trials more efficientThe evidence base for how to make the trials process
efficient is remarkably thin. Trial Forge aims to change this.Explore Pathway Learn More
Trials
Randomised controlled trials are the gold standard for evaluating healthcare treatments; 1000s are done every year.
Essential
Randomised trials are the cornerstone of evidence-based healthcare because they offer the fairest tests of treatments, therapies and initiatives.
Inefficient
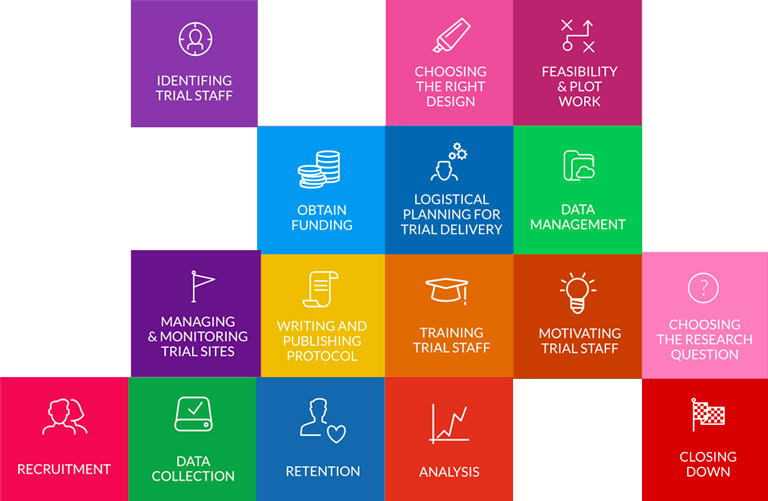
The evidence base for how to make the trials process efficient is remarkably thin. Trial Forge aims to change this.
Small Changes add up
Trial Forge will make trials more efficient by looking for marginal gains across all trial processes, from research question to implementation into routine care. It will encourage everyone connected with trials to be more sceptical of what we do by asking for the evidence behind all of our trial decisions.
LATEST NEWS & EVENTS
A list of 11 priority recruitment and retention SWATs
Resources to help with the design and delivery of SWATs
LATEST RESOURCES
Guidance– Trial Forge Guidance 3: randomised trials and how to recruit and retain individuals from ethnic minority groups—practical guidance to support better practice
Randomised trials, especially those intended to directly inform clinical practice and policy, should be designed to reflect all those who
- Resource Type: Guidance
- Date Posted:
Brief guidance– Enhancing Communication in Clinical Trials: The TMRN-TMRP Communication Wheel
Frances Shiely, Ratna Sohanpal, Andrew Willis, Talia Isaacs, Julia Wade, Vincent Russell, Shaun Treweek, Annabelle South, on behalf of the
- Resource Type: Brief guidance
- Date Posted:
Leaflet– Pam dylwn i ystyried gwreiddio SWAT yn ein treial?
Byrfodd Saesneg am ‘Study Within A Trial’ (Astudiaeth o fewn Treial) yw SWAT ac mae’n astudiaeth ymchwil sy’n cael ei
- Resource Type: Leaflets
- Date Posted: